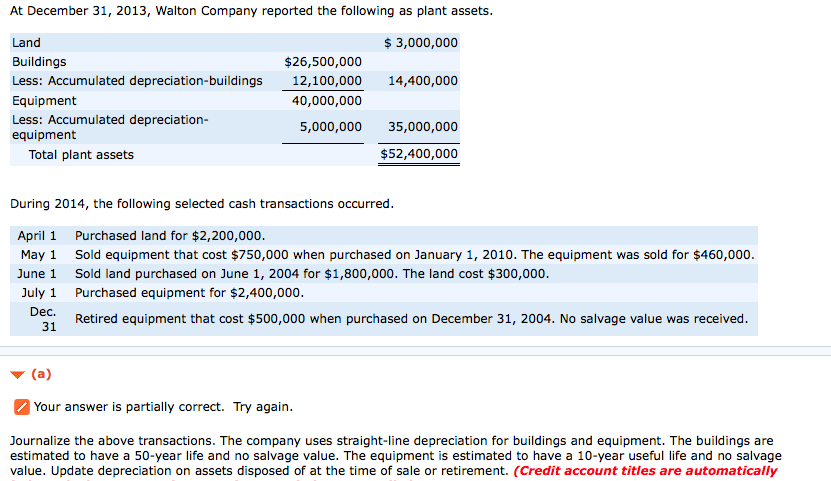
40K decays to 40Ar with a half-life of 1.25 x 109 yr. Assume that rocks contain no 40Ar when they form, and that the only way 40Ar can be present is through the decay of 40K. If the ratio of 40K to 40Ar in a particular rock is found to be 1:7, what is the age of the rock.
Learning Objectives
- Define half-life.
- Determine the amount of radioactive substance remaining after a given number of half-lives.
Whether or not a given isotope is radioactive is a characteristic of that particular isotope. Some isotopes are stable indefinitely, while others are radioactive and decay through a characteristic form of emission. As time passes, less and less of the radioactive isotope will be present, and the level of radioactivity decreases. An interesting and useful aspect of radioactive decay is half-life, which is the amount of time it takes for one-half of a radioactive isotope to decay. The half-life of a specific radioactive isotope is constant; it is unaffected by conditions and is independent of the initial amount of that isotope.
Peer Review is an upcoming total conversion mod by PSR Digital that aims to reboot Half-Life: Decay using the 2013MP branch of the Source engine. 1 Overview 2 Details 3 Gallery 3.1 Screenshots 3.2 Models 3.3 Concept Art 3.4 Video 4 External links Peer Reviewis a reboot ofHalf-Life's third and final expansion,Half-Life: Decay. Return to the Black Mesa Research Facility and relive the chaos from. Example of the Half-Year Convention. As an example, assume a company purchases a $105,000 delivery truck with a salvage value of $5,000 and an expected life of 10 years. Oct 06, 2008 25 digit cd key for counter strike condition zero All About Music And Technology. 5zc2f - kzzr7 - 4527r - f26lw - vr2wb. Posted by Vijay Bhasker at 2:37 AM. Newer Post Older Post Home.
Consider the following example. Suppose we have 100.0 g of tritium (a radioactive isotope of hydrogen). It has a half-life of 12.3 y. After 12.3 y, half of the sample will have decayed from hydrogen-3 to helium-3 by emitting a beta particle, so that only 50.0 g of the original tritium remains. After another 12.3 y—making a total of 24.6 y—another half of the remaining tritium will have decayed, leaving 25.0 g of tritium. After another 12.3 y—now a total of 36.9 y—another half of the remaining tritium will have decayed, leaving 12.5 g. This sequence of events is illustrated in Figure 15.1 “Radioactive Decay”.
Figure 15.1 Radioactive Decay
During each successive half-life, half of the initial amount will radioactively decay.
We can determine the amount of a radioactive isotope remaining after a given number half-lives by using the following expression:
where n is the number of half-lives. This expression works even if the number of half-lives is not a whole number.
Example 3
The half-life of fluorine-20 is 11.0 s. If a sample initially contains 5.00 g of fluorine-20, how much remains after 44.0 s?
Solution
If we compare the time that has passed to the isotope’s half-life, we note that 44.0 s is exactly 4 half-lives, so using the previous expression, n = 4. Substituting and solving results in the following:
Less than one-third of a gram of fluorine-20 remains.
Test Yourself

The half-life of titanium-44 is 60.0 y. A sample of titanium contains 0.600 g of titanium-44. How much remains after 240.0 y?
Answer
0.0375 g
Half-lives of isotopes range from fractions of a microsecond to billions of years. Table 15.2 “Half-Lives of Various Isotopes” lists the half-lives of some isotopes.
Table 15.2 Half-Lives of Various Isotopes
| Isotope | Half-Life |
|---|---|
| 3H | 12.3 y |
| 14C | 5730 y |
| 40K | 1.26 × 109 y |
| 51Cr | 27.70 d |
| 90Sr | 29.1 y |
| 131I | 8.04 d |
| 222Rn | 3.823 d |
| 235U | 7.04 × 108 y |
| 238U | 4.47 × 109 y |
| 241Am | 432.7 y |
| 248Bk | 23.7 h |
| 260Sg | 4 ms |
Chemistry Is Everywhere: Radioactive Elements in the Body
You may not think of yourself as radioactive, but you are. A small portion of certain elements in the human body are radioactive and constantly undergo decay. The following table summarizes radioactivity in the normal human body.
| Radioactive Isotope | Half-Life (y) | Isotope Mass in the Body (g) | Activity in the Body (decays/s) |
|---|---|---|---|
| 40K | 1.26 × 109 | 0.0164 | 4,340 |
| 14C | 5,730 | 1.6 × 10−8 | 3,080 |
| 87Rb | 4.9 × 1010 | 0.19 | 600 |
| 210Pb | 22.3 | 5.4 × 10−10 | 15 |
| 3H | 12.3 | 2 × 10−14 | 7 |
| 238U | 4.47 × 109 | 1 × 10−4 | 5 |
| 228Ra | 5.76 | 4.6 × 10−14 | 5 |
| 226Ra | 1,620 | 3.6 × 10−11 | 3 |
The average human body experiences about 8,000 radioactive decays/s.
Most of the radioactivity in the human body comes from potassium-40 and carbon-14. Potassium and carbon are two elements that we absolutely cannot live without, so unless we can remove all the radioactive isotopes of these elements, there is no way to escape at least some radioactivity. There is debate about which radioactive element is more problematic. There is more potassium-40 in the body than carbon-14, and it has a much longer half-life. Potassium-40 also decays with about 10 times more energy than carbon-14, making each decay potentially more problematic. However, carbon is the element that makes up the backbone of most living molecules, making carbon-14 more likely to be present around important molecules, such as proteins and DNA molecules. Most experts agree that while it is foolhardy to expect absolutely no exposure to radioactivity, we can and should minimize exposure to excess radioactivity.
What if the elapsed time is not an exact number of half-lives? We can still calculate the amount of material we have left, but the equation is more complicated. The equation is
where e is the base of natural logarithms (2.71828182…), t is the elapsed time, and t1/2 is the half-life of the radioactive isotope. The variables t and t1/2 should have the same units of time, and you may need to make sure you know how to evaluate natural-logarithm powers on your calculator (for many calculators, there is an “inverse logarithm” function that you can use; consult your instructor if you are not sure how to use your calculator). Although this is a more complicated formula, the length of time t need not be an exact multiple of half-lives.
Example 4
The half-life of fluorine-20 is 11.0 s. If a sample initially contains 5.00 g of fluorine-20, how much remains after 60.0 s?
Solution
Although similar to Example 3, the amount of time is not an exact multiple of a half-life. Here we identify the initial amount as 5.00 g, t = 60.0 s, and t1/2 = 11.0 s. Substituting into the equation:
amount remaining = (5.00 g) × e−(0.693)(60.0 s)/11.0 s
Evaluating the exponent (and noting that the s units cancel), we get
Half Life 1 Cd Key 25 Digits
amount remaining = (5.00 g) × e−3.78
Solving, the amount remaining is 0.114 g. (You may want to verify this answer to confirm that you are using your calculator properly.)
Test Yourself
The half-life of titanium-44 is 60.0 y. A sample of titanium contains 0.600 g of titanium-44. How much remains after 100.0 y?
Answer
0.189 g
Key Takeaways
- Natural radioactive processes are characterized by a half-life, the time it takes for half of the material to decay radioactively.
- The amount of material left over after a certain number of half-lives can be easily calculated.
Exercises
Do all isotopes have a half-life? Explain your answer.
Which is more radioactive—an isotope with a long half-life or an isotope with a short half-life?
How long does it take for 1.00 g of palladium-103 to decay to 0.125 g if its half-life is 17.0 d?
How long does it take for 2.00 g of niobium-94 to decay to 0.0625 g if its half-life is 20,000 y?
It took 75 y for 10.0 g of a radioactive isotope to decay to 1.25 g. What is the half-life of this isotope?
It took 49.2 s for 3.000 g of a radioactive isotope to decay to 0.1875 g. What is the half-life of this isotope?
The half-live of americium-241 is 432 y. If 0.0002 g of americium-241 is present in a smoke detector at the date of manufacture, what mass of americium-241 is present after 100.0 y? After 1,000.0 y?
If the half-life of tritium (hydrogen-3) is 12.3 y, how much of a 0.00444 g sample of tritium is present after 5.0 y? After 250.0 y?
Explain why the amount left after 1,000.0 y in Exercise 7 is not one-tenth of the amount present after 100.0 y, despite the fact that the amount of time elapsed is 10 times as long.
Explain why the amount left after 250.0 y in Exercise 8 is not one-fiftieth of the amount present after 5.0 y, despite the fact that the amount of time elapsed is 50 times as long.
An artifact containing carbon-14 contains 8.4 × 10−9 g of carbon-14 in it. If the age of the artifact is 10,670 y, how much carbon-14 did it have originally? The half-life of carbon-14 is 5,730 y.
Carbon-11 is a radioactive isotope used in positron emission tomography (PET) scans for medical diagnosis. Positron emission is another, though rare, type of radioactivity. The half-life of carbon-11 is 20.3 min. If 4.23 × 10−6 g of carbon-11 is left in the body after 4.00 h, what mass of carbon-11 was present initially?

Answers
1.
Only radioactive isotopes have a half-life.

3.
51.0 d
5.
25 y
7.
0.000170 g; 0.0000402 g
9.
Radioactive decay is an exponential process, not a linear process.
11.
3.1 × 10−8 g
The following tools can generate any one of the values from the other three in the half-life formula for a substance undergoing decay to decrease by half.
Half-Life Calculator
Please provide any three of the following to calculate the fourth value.
Half-Life, Mean Lifetime, and Decay Constant Conversion
Please provide any one of the following to get the other two.
Definition and Formula
Half-life is defined as the amount of time it takes a given quantity to decrease to half of its initial value. The term is most commonly used in relation to atoms undergoing radioactive decay, but can be used to describe other types of decay, whether exponential or not. One of the most well-known applications of half-life is carbon-14 dating. The half-life of carbon-14 is approximately 5,730 years, and it can be reliably used to measure dates up to around 50,000 years ago. The process of carbon-14 dating was developed by William Libby, and is based on the fact that carbon-14 is constantly being made in the atmosphere. It is incorporated into plants through photosynthesis, and then into animals when they consume plants. The carbon-14 undergoes radioactive decay once the plant or animal dies, and measuring the amount of carbon-14 in a sample conveys information about when the plant or animal died.
Below are shown three equivalent formulas describing exponential decay:
- where
N0 is the initial quantity
Nt is the remaining quantity after time, t
t1/2 is the half-life
τ is the mean lifetime
λ is the decay constant
Half Life 25 Digit Code
If an archaeologist found a fossil sample that contained 25% carbon-14 in comparison to a living sample, the time of the fossil sample's death could be determined by rearranging equation 1, since Nt, N0, and t1/2 are known.
This means that the fossil is 11,460 years old.
Derivation of the Relationship Between Half-Life Constants
Half Life Cd Key 25 Digits
Using the above equations, it is also possible for a relationship to be derived between t1/2, τ, and λ. This relationship enables the determination of all values, as long as at least one is known.